
Rubber tire characterization is a common analysis performed by tire manufacturers to ensure the quality of their products. Rubber tire composition is often complex and frequently includes components from several sources and suppliers. The formulation details of the final products are often not known to the manufacturer. Companies in later stages in the supply chain may have even less information on components in the final formulations. Therefore, the same part number at a point in the supply chain can result in a polymer part not made with the same formulation, yet the apparent polymer properties may seem equivalent. After usage by a company or customer, a deformulation analysis may be required to determine the products’ detailed chemical composition.
This article illustrates the power and simplicity of modern-day pyrolysis-GC/MS in a rubber tire’s material characterization. Using the modern multi-functional micro-furnace pyrolyzer directly interfaced to a GC, the detailed chemical composition of different parts in a tire is identified.
Experimental: Rubber samples were collected from the (a) tread, (b) shoulder, (c) sidewall, and (d) bead parts of an automobile tire. Each sample was cut into approximately 1 mm squares. The Double-Shot mode of operation was used to thermally extract additives from polymeric/heavier fractions of the samples. With this mode of operation, a sample is analyzed twice; first, volatiles and additives are analyzed, and then the polymeric/heavier portion of the sample.
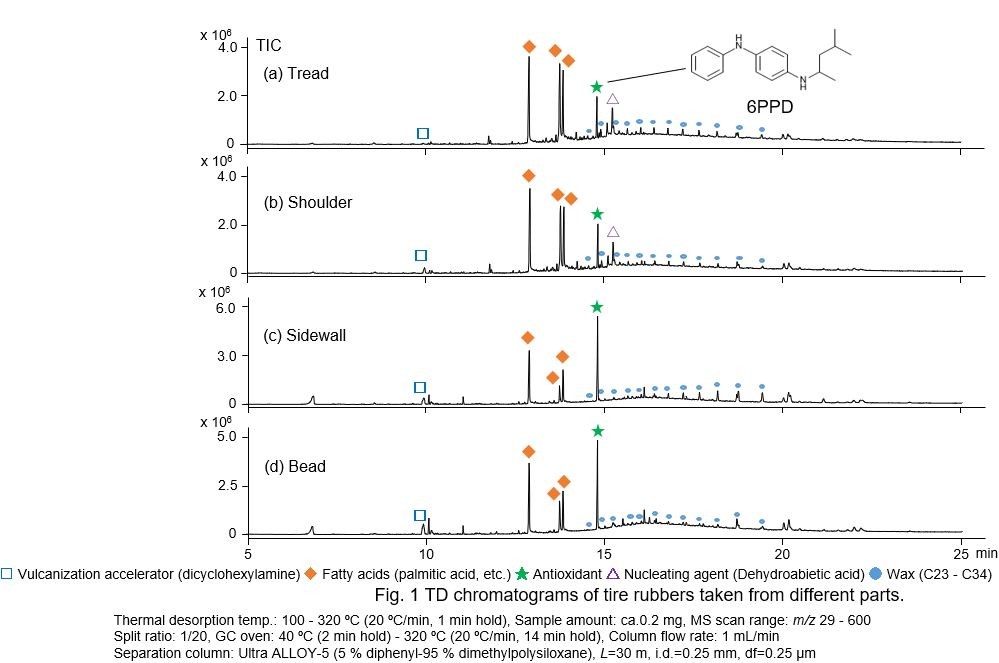
For Thermal Desorption analysis (first-shot), the pyrolyzer furnace was temperature-programmed from 100 to 320C (20C/min). The gases evolved from the sample were identified as various additives using GC/MS. From Figs. 1 (a) and (b), vulcanization accelerators, fatty acids, antiaging agents, nucleating agents, waxes, etc., are identified. In Figs. 1 (c) and (d), the additives present in (a) and (b) are similarly detected, but no nucleating agent is detected. 6PPD contents in the tread, shoulder, sidewall, and bead were also calculated as1452 ppm, 1744 ppm, 6122 ppm, and 4868 ppm, respectively (RSD <5 %, n = 3).
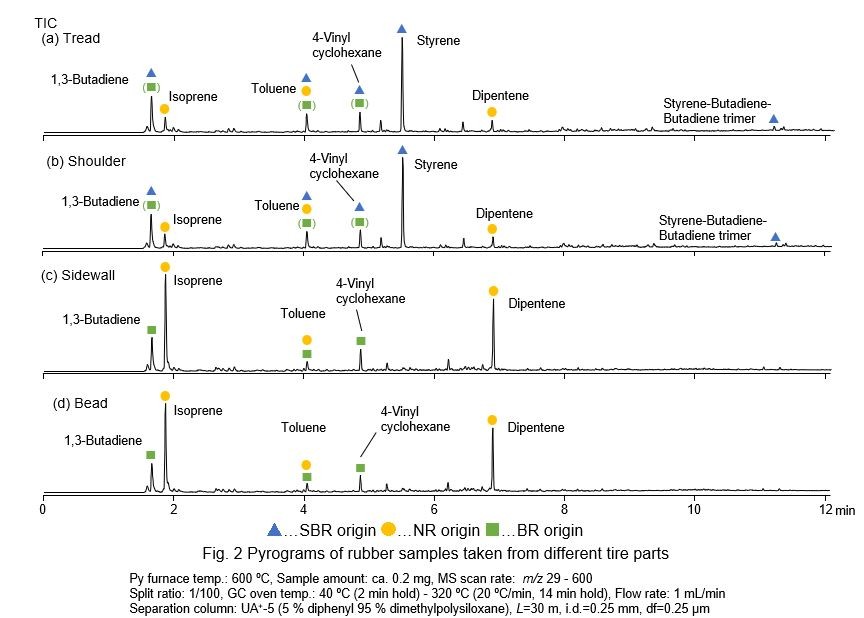
In Figs. 2 (a) and (b), strong peaks of styrene and 1,3-butadiene are recognized, which are originated from styrene-butadiene-rubber (SBR). Peaks of isoprene and dipentene originated from natural rubber (NR), is intensified in Figs. 2 (c) and (d), suggesting that NR is the major constituent in sidewall and bead. In addition, peaks of 1,3-butadiene and 4-vinylbenzene can be related to butadiene rubber (Frontier Laboratories, Ltd.).
References: This article is based on a technical note developed by Frontier Laboratories Ltd. 4-16-20 Saikon, Koriyama, Fukushima, 963-8862 JAPAN. www.frontier-lab.com.
Flexible financing, technical services, and refurbished instruments.
Everything you need to advance your lab’s success – all in one place.
8301 New Trails Drive, Suite 100, The Woodlands, Texas 77381
Complete this form below to sign up and we will reach out to you with instructions
