
On the quest to achieve accurate quantification of CO, CO2, formic acid, formaldehyde, and formamide, greater response from gas chromatography is often needed. The flame ionization detector (FID) is widely used in the field of gas chromatography because it is highly sensitive to many organic compounds and provides a linear response over many orders of magnitude.
However, FIDs suffer from two main drawbacks: the response to analytes are variable (and therefore require time-consuming calibration) and the FID provides low or no response to highly-functionalized molecules. In this application note, we show that an FID equipped with a Polyarc® reactor is not only highly sensitive to these compounds but also provides a response factor that is equivalent for all carbon-containing compounds, thus eliminating the need for time-consuming calibration.
Compared to conventional FID-only systems, the Polyarc® reactor eliminates the need for a second detector to quantify carbon monoxide and carbon dioxide, improves accuracy in quantitative analysis, and saves time and money associated with calibrations.
Accurate quantification of CO and other molecules by GC/FID is often a time-consuming process, in part, because the response factors for every analyte must be determined before quantitative results can be obtained. Response factors (RF) correct the detector signal for differences in detector—analyte sensitivity and are typically defined with respect to the response of another molecule (i.e., the internal standard) as shown below:
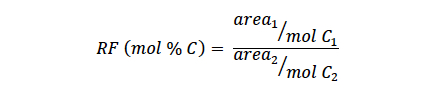
Where (1) and (2) refer to the analyte and internal standard, respectively, area is the GC/FID peak area (i.e., the integrated detector response), and mol C are the injected moles of carbon of the component (the concentration of the component in terms of carbon content in the sample could also be used).
Response factors in typical GC/FID analyses are dependent on the chemical structure of the molecule. Heteroatoms such as O, N, P, S, and Cl decrease the response of a given analyte in an FID detector, thus necessitating calibration. The reason for the decreased response factor is the diminished presence of CH and related hydrocarbon radicals. Ultimately, hydrocarbon radicals are thought to react with an oxygen atom to form the CHO+ ion according to the following reaction:¹
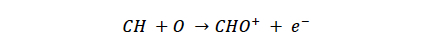
The CHO+ ion is responsible for the measured signal in the FID detector. Without the presence of CH, related hydrocarbon radicals, and ultimately the CHO+ ion, there is no FID signal. This explains the experimental observation that molecules lacking C-H bonds such as carbon dioxide (CO2) and carbon monoxide (CO) are not detectable by conventional FID detectors.
Conversely, the Polyarc® reactor converts all organic compounds to methane prior to detection in the FID according to the following basic reaction:
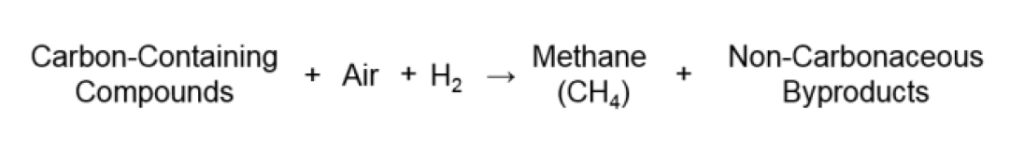
Since all carbon-containing compounds are converted to methane, the response of the FID is equivalent for all molecules on a per-carbon atom basis and previously undetectable molecules such as carbon monoxide and carbon dioxide are easily quantified. Thus the response factors, as defined above, become unity for all carbon-containing molecules.
In this work, we demonstrate how to achieve accurate quantitative analysis of CO, CO2, formic acid, formaldehyde, and formamide using an FID equipped with a Polyarc® reactor. The results show that the Polyarc® setup allows for the sensitive analysis of these molecules (<30 ppm) without calibrations and in the presence of numerous other molecules.
An Agilent 7890A GC equipped with a split/splitless inlet, a Polyarc® reactor (ARC PA-RRC-A02) was used for the analysis. Air (zero grade, Praxair) and H2 (99.999%, Praxair) were supplied to the FID and to the ARC manual flow control module (PA-CAS-A07). Helium (99.999%, Praxair) was used as the carrier gas.
The system was configured with the column connected from the split/splitless inlet to the Polyarc®/FID and to the FID-only.
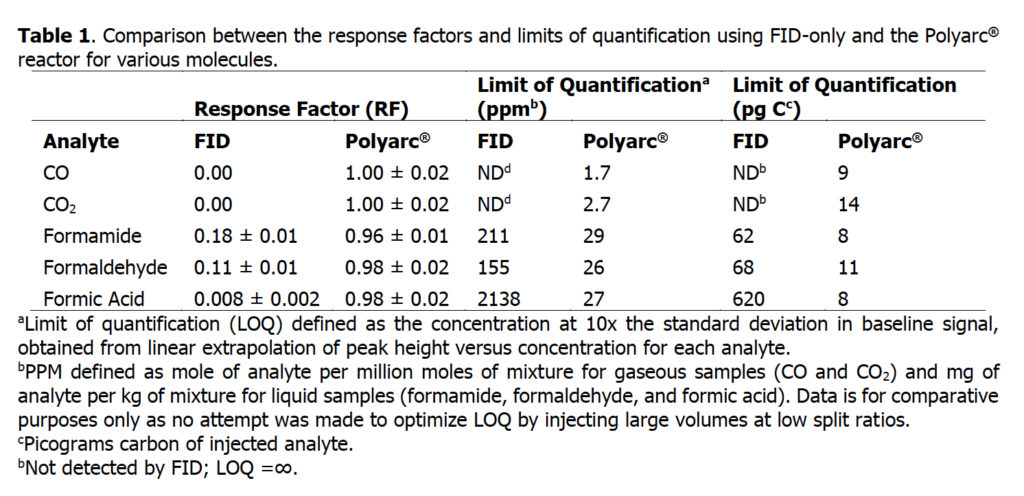
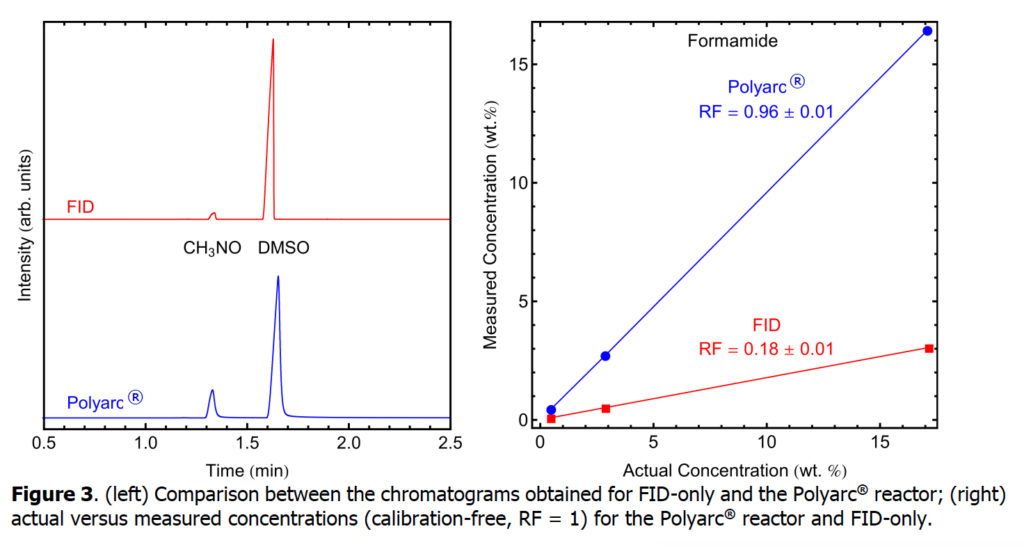
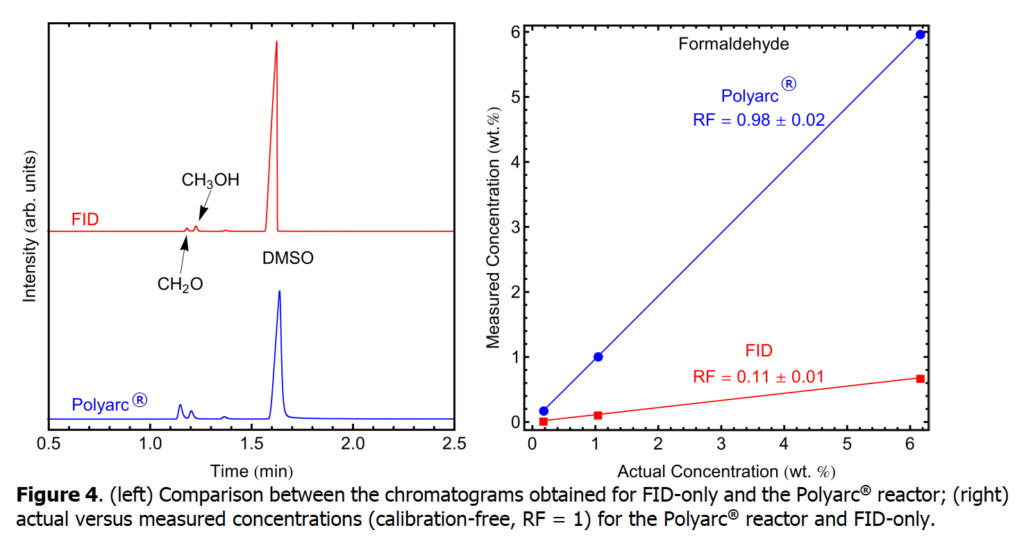
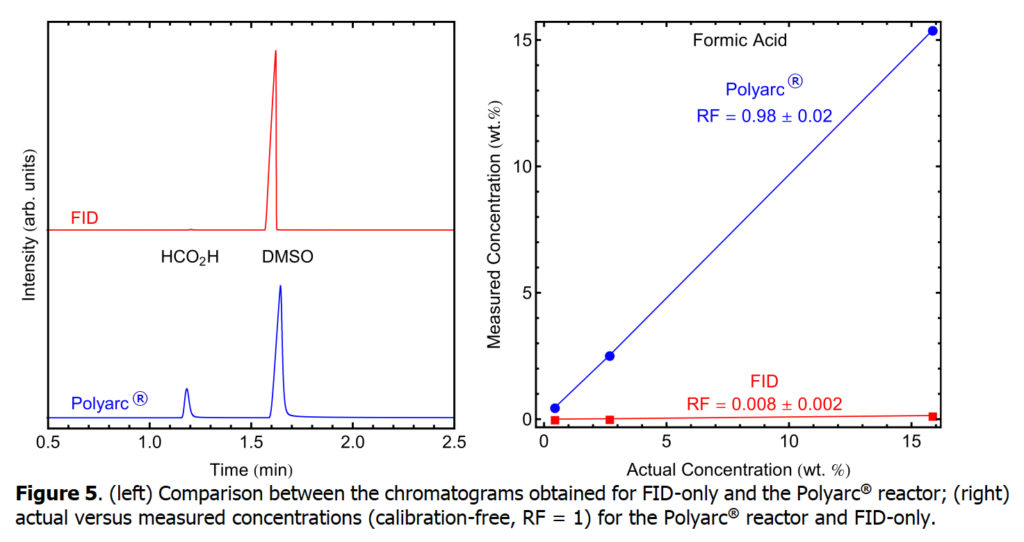
The response factors for five compounds that typically have low response or no response in FID detectors were determined with a conventional FID-only system and compared with the Polyarc® reactor. A bar chart comparing the response factors for FID-only and the Polyarc® reactor is shown in Figure 1 with the tabulated results presented in Table 1. The response factors for all compounds tested with the Polyarc® reactor are 1. CO and CO2 showed no response in the FID-only system. The Polyarc® reactor is ~100 x more sensitive than FID-only to formic acid, ~10 x more sensitive to formaldehyde, and ~5 x more sensitive to formamide.
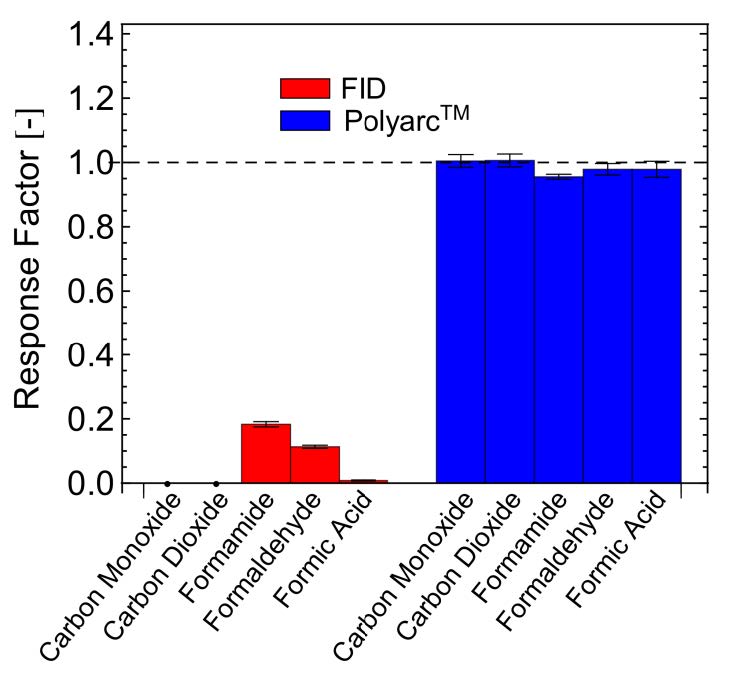
Figure 1 – Comparison between the response factors using FID-only and the Polyarc® reactor for compounds that have very low responses in FIDs.
Chromatograms obtained for FID-only and the Polyarc® reactor are presented below for each of the compounds in Table 1. Furthermore, parity plots comparing the actual concentrations and measured concentrations assuming RF =1 are presented, showing the linearity of the Polyarc® reactor across a range of concentrations and the increased response over FID-only.
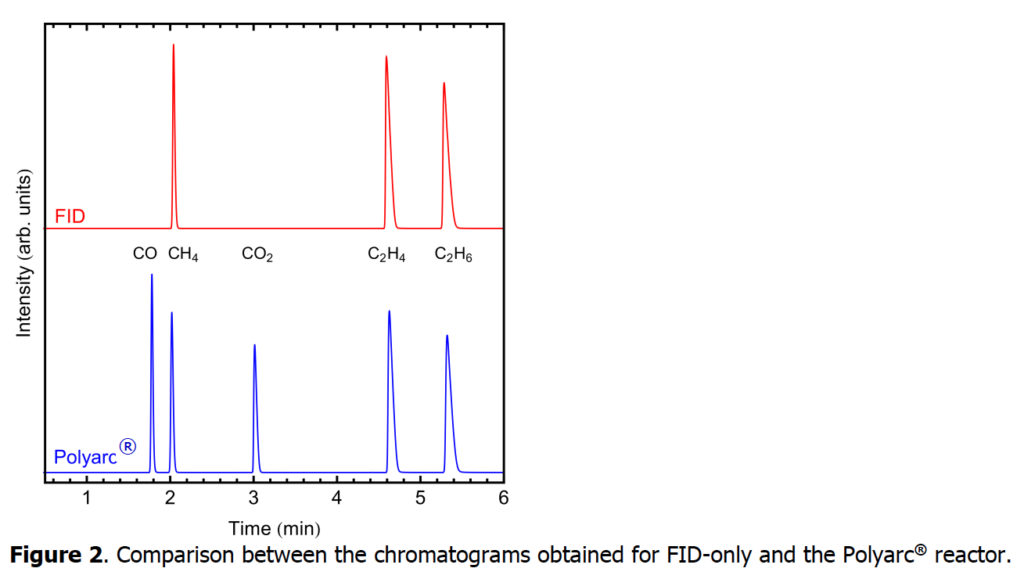
First, we explore the quantification of carbon monoxide and carbon dioxide using an FID-only setup and compare it with the Polyarc + FID setup.
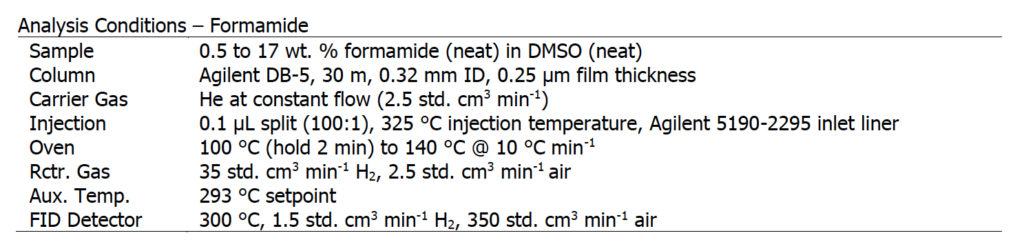
Second, we explore the analysis of formamide using an FID-only setup and compare it with the Polyarc + FID setup. Formamide is an amide derived from formic acid. It is a colorless liquid which is miscible with water and has an ammonia-like odor.
Note – DMSO should not be injected with larger injection volumes or with lower split ratios than those shown below to prevent sulfur poisoning.
Next, we explore the analysis of formaldehyde using an FID-only setup and compare it with the Polyarc + FID setup. Formaldehyde is a naturally occurring organic compound with the formula CH2O (H−CHO).
Note – DMSO should not be injected with larger injection volumes or with lower split ratios than those shown below to prevent sulfur poisoning.
Next, we explore the analysis of formic acid using an FID-only setup and compare it with the Polyarc + FID setup. Formic acid, systematically named methanoic acid, is the simplest carboxylic acid, and has the chemical formula H2CO2. It is an important intermediate in chemical synthesis and occurs naturally.
Note – DMSO should not be injected with larger injection volumes or with lower split ratios than those shown below to prevent sulfur poisoning.

The Polyarc® reactor is highly sensitive and provides a uniform response to compounds that are undetectable in standard FID detectors such as carbon monoxide and carbon dioxide. Furthermore, the Polyarc® reactor is also highly sensitive to compounds that have a very low response in FIDs such as formic acid, formaldehyde, and formamide. The sensitivity and robustness of the Polyarc® system mark a first of its kind in the field of analytical chemistry – other reactor-based technologies such as methanizers are incapable of producing a high and uniform response to all organic molecules.
In summary, the Polyarc® reactor is game changer when it comes to accurate quantification of CO, CO2, and other molecules that have no or low response in traditional FID detectors. The Polyarc saves time, improves analysis sensitivity and accuracy, and eliminates the need for a second detector.
To purchase a Polyarc system, please contact Quantum Analytics.
Flexible financing, technical services, and refurbished instruments.
Everything you need to advance your lab’s success – all in one place.
8301 New Trails Drive, Suite 100, The Woodlands, Texas 77381
Complete this form below to sign up and we will reach out to you with instructions
