
Microplastics research is a rapidly advancing field due to the widespread presence of these contaminants and their potential health risks. Chemists play a crucial role in analyzing microplastics from various environmental matrices like water, soil, and biota. Pyrolysis GC/MS offers a powerful analytical technique for microplastic identification and quantification, making it an invaluable research tool.
The study of microplastics is not only a rigorous, tenured study but also a critical and fervent one. Researchers continue to find traces of plastic in the environments we live in and work to understand their consequential effects on our bodies. Analytical techniques, such as Pyrolysis GC/MS, provides chemists with nuanced insights into the extent of the problem by enabling more accurate assessments and solutions. This article delves into the microplastics lab to explore Pyrolysis GC/MS as a method used in the analysis of trace plastics.

Pyrolysis GC/MS is known for its unique advantages in microplastics analysis:

How do we introduce microplastics, or even plastics, to a gas chromatography system? The Frontier 3030D Multi-Shot Pyrolyzer is a furnace that sits above the GC inlet. This microfurnace can heat to high temperatures, well above that of an inlet, breaking down microgram amounts of polymer samples into volatile pyrolyzates for GC analysis.
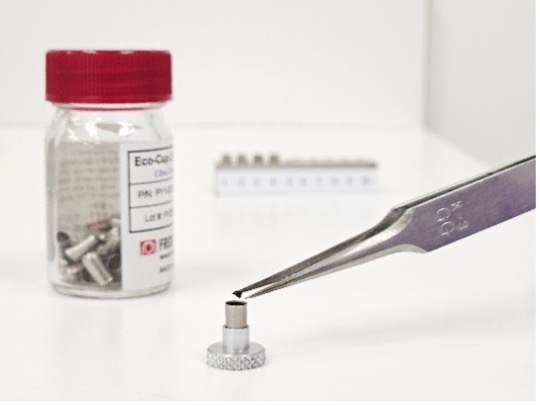
Microplastic analysis presents unique challenges due to their small size, diverse polymer types, and presence within complex matrices. Efficient sample preparation is crucial for concentrating microplastics and minimizing matrix interference.
There are a few types of matrices to consider: tissue/biomatter, soil/sediment, and air/water.
Techniques include:
Tissues such as seafood, poultry, or even human tissue are digested with an appropriate solvent that dissolves the tissue, but not the polymer. Sodium hydroxide, sodium hypochlorite, acids, and enzymes are all digesters that have been used in this type of analysis. The same solvent that digests tissue may not digest starches or plant cellulose.
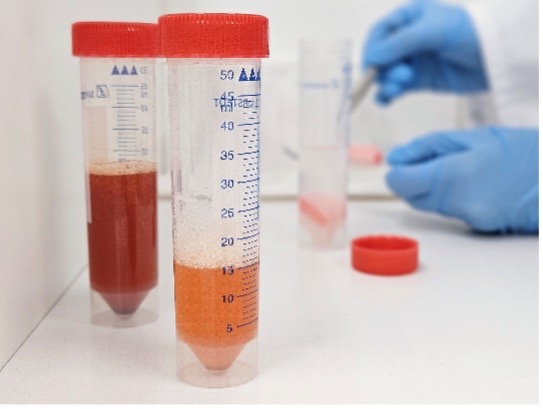
Microplastics in sediment are separated by density. Sediment is placed in water and agitated. Once the sediment settles, many plastics float as they are less dense than water. However, some polymer types are heavier or equal to the density of water. These polymers sink with the sediment or are suspended in water, making them difficult to collect.

Saturating the water with salt increases the density of water allowing dense polymers to float. Heavier salts such as sodium iodide (NaI) or zinc chloride (ZnCl2) can be used to markedly increase the density of water, allowing a greater range of polymer types to float. Once the sediment settles, the water is vacuum-filtered for analysis.

A key detail in pyrolysis sample preparation is the use of quartz or glass filters. Quartz does not break down in the pyrolyzer with the polymers. This means your chromatogram will ideally consist of pyrolyzates from the polymers of interest, and NOT the matrix nor your sample preparation material.

In fact, extracting microplastics from water or air can be as simple as running or pumping water or air through a glass filter (check out this microplastics in air white paper). Tissue digestates and water from a density separator are also vacuum filtered through glass filters. The filters can then be whole punched, cut or, ideally, cryo-milled to be placed into the Eco-Cup for analysis.

Before any samples are run, a calibration is required.
A standard composed of known polymers are weighed at different amounts to build a curve. Frontier’s calibration kit includes 12 of the most common polymers in SiO2 and CaCO3. Calibrating is as easy as weighing increasing milligram amounts of the calibrant into different sample cups. A 4mg of standard, for example, will contain microgram amounts of each of the 12 polymers. This makes is easier to weigh out small amounts of polymer easy to do, even without a microbalance. Additionally, even smaller amounts can be weighed by “diluting” the standard with more SiO2 and CaCO3 included in the calibration kit.
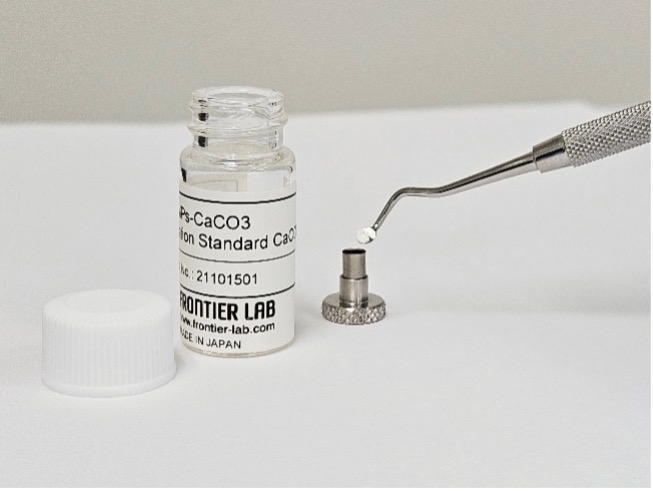
Calibration with standard mixtures containing known polymers is essential for quantifying microplastics in your samples. Software like F-Search MP 2.1 streamlines data processing by facilitating curve generation for standard polymers and identifying polymers within your samples. It also provides quantitative data, like weight percentages of each polymer type and identification confidence levels. Additionally, the software allows for qualitative analysis by searching against libraries of additives and other polymers for a more comprehensive understanding of the microplastic composition.

Pyrolysis GC/MS is at the frontlines of microplastics research thanks to its unique capabilities. It continues to be a valuable tool for chemists investigating microplastic contamination due to its ability to differentiate polymers, detect additives, and provide quantitative data. This technique plays a vital role in advancing microplastics research and understanding of their environmental impact.
Ready to enhance your microplastics analysis capabilities? Quantum Analytics offers comprehensive solutions and technical services for those looking to implement Pyrolysis GC/MS instrumentation. By partnering with Quantum Analytics, researchers and laboratories can leverage the full potential of this cutting-edge technology to expand their applications and drive impactful research. Contact us today to get started.
Flexible financing, technical services, and refurbished instruments.
Everything you need to advance your lab’s success – all in one place.
8301 New Trails Drive, Suite 100, The Woodlands, Texas 77381
Complete this form below to sign up and we will reach out to you with instructions
